On December 19, 2016 the U.S. Food and Drug Administration granted accelerated approval to Rubraca (rucaparib) to treat women with a certain type of ovarian cancer. Rubraca is approved for women with advanced ovarian cancer who have been treated with two or more chemotherapies and whose tumors have a specific gene mutation (deleterious BRCA) as identified by an FDA-approved companion diagnostic test.
The structure of Rucaparib is given below:
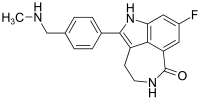
Rubraca is marketed by Clovis Oncology, Inc. based in Boulder, Colorado. The FoundationFocus CDxBRCA companion diagnostic is marketed by Foundation Medicine, Inc. of Cambridge, Massachusetts.
Rucaparib is claimed as a product in US 6,495,541 B1 which is set to expire in January 2020. However, the patent may get patent term extension.
US ‘541 issued to Agouron Pharmaceuticals which was acquired by Warner-Lambert. Later, in June 2000, Warner-Lambert merged with Pfizer.
Clovis Oncology entered into an agreement with Pfizer for the development and commercialisation of Rucaparib. Under the terms of the agreement, Clovis is responsible for global development and commercialisation of Rucaparib.
