In Mylan v. Aurobindo the Federal Circuit affirms the grant of a preliminary injunction based upon the infringement of US 9,353,050 which is the one of the three patents in suit.
The panel reverses the injunction to the other two process patents based on the district court err in analyzing the doctrine of equivalents under the “function, way, result” test (FWR test)
Background:
the product Isosulfan blue (ISB)developed by Hirsch Industries as a 1% injectable solution which was commercialized under the trade name Lymphazurin. However, the production of ISB had been plagued by difficulties in synthesizing and purifying ISB.
The analysis of Hirsch’s ISB revelas that it contains 94.5% ISB as determined by HPLC, with the remaining 5.5% consisting of “closely related isomers” produced during synthesis.
For 26 years following the Food and Drug Administration’s(“FDA”) approval of ISB, Sigma-Aldrich Corp supplied Hirsch and its successors with ISB that was manufactured by Allied Chemical Corp. (“Allied”). Allied’s manufacturing process was unknown, but analysis of its ISB indicated the presence of lead, which suggested the use of a lead compound in synthesis. Sigma developed an isolation process to remove the unwanted lead, but the ultimate purity of the ISB it sold was unknown.
In 2000, Allied stopped supplying Sigma with ISB and, while Sigma was looking for a new supplier, Covidien was forced to notify its customers that it was “completely out of” Lymphazurin® until it could find a new supplier for ISB. By 2008, Sigma had a new supplier, Innovassynth, which synthesized ISB using ammonium dichromate, resulting in residual chromium impurities. Sigma reported numerous problems with the purity of Innovassynth’s product and eventually developed its own manufacturing process for ISB sometime around 2010.
Aurobindo’s ANDA:
Aurobindo sought FDA approval for a generic Lymphazurin®, informing the FDA that it had studied a “number of patents” describing ISB manufacture and selected, inter alia, Apicore’s U.S. Patent 7,662,992 , and that it “considered the process described [therein] for the initial sample preparation and further, the optimization of the process.” Id. (internal quotation marks omitted). Aurobindo acknowledged to the FDA that it was looking for a reagent “other than silver oxide.” Id. (internal quotation marks omitted). It eventually selected manganese dioxide, and its process resulted in ISB with a 5–10% impurity which could not be removed by recrystallization. Instead, it used preparatory HPLC to achieve an ISB purity of greater than 99.5%. Mylan sued Aurobindo for infringement and sought a preliminary injunction, which the district court granted.
Apicore and its patent portfolio:
Apicore was founded in 2003 and began developing an improved process for synthesizing ISB. In 2004, Apicore partnered with Synerx Pharma LLC (“Synerx”), Mylan Inst.’s predecessor, to develop and market a generic version of Lymphazurin®. In 2007, Apicore filed a patent application that ultimately led to the process and ’050 patents. Based on the claimed process, Synerx (acquired by Mylan Inst. in 2012) filed an abbreviated new drug application (“ANDA”) seeking FDA approval to market a generic Lymphazurin®; the FDA approved the ANDA in 2010. By 2011, ISB sales were a significant portion of Apicore’s revenue and in 2012, Covidien withdrew Lymphazurin® from the market for “reasons other than safety or effectiveness.” Mylan Inst. became the sole supplier of the 1% ISB drug product until 2016, when Aurobindo entered the market.
Apicore owns, and Mylan Inst. is the exclusive licensee of, the ’992, ’616, and ’050 patents, which relate to ISB, a triarylmethane dye used to map lymph nodes. The ’992 and ’616 patents (together, “the process patents”) are directed to a process for preparing ISB by reacting isoleuco acid with silver oxide in a polar solvent, followed by reaction with a sodium solution. See, e.g., ’992 patent col. The ’992 patent further requires 2.0–3.0 equivalents of silver oxide.
A brief overview of Apicore patents is given below:
|
U.S. Patent 7,662,992 (Expiry: May 11, 2027) |
U.S. Patent 8,969,616 (Expiry: May 11, 2027) | U.S. Patent 9,353,050 (Expiry: December 15, 2028) |
| Claims a process for preparing Isosulfan blue sodium salt which comprises treating isoleuco acid with 2.0 to 3.0 equivalents of silver oxide in a polar solvent | Claims a process for the preparation of Isosulfan blue sodium salt which is similar to the process claimed in US ‘992
|
Claims Isosulfan blue sodium having a purity of at least 99.0% by HPLC
|
The district court evaluated the likelihood of success on the merits and found that Aurobindo likely infringed the process patents under the doctrine of equivalents.
The district court found that the difference in oxidation strength between silver oxide and manganese dioxide is “irrelevant” under both the “function-way-result” (“FWR”) and “insubstantial differences” tests for equivalence, as applied to the “face of the claims,” because the claims do not specify a requirement of oxidation strength.
The district court also rejected Aurobindo’s obviousness argument, finding that Aurobindo did not raise a substantial question regarding motivation to combine the references or a reasonable expectation of success. Aurobindo appeals the district court’s grant of a preliminary injunction.
The circuit reverses the district court’s grant of the preliminary injunction against the process patents by stating that Manganese dioxide and silver oxide are substantially different in many respects. For example, manganese and silver are in different groups of the Periodic Table. In oxide form, manganese has an oxidation state of +4, while silver is +1. Those differences may well be relevant to equivalence at trial. Thus, the choice of test under the doctrine of equivalents may matter in this case.
With respect to US ‘050 patent Aurobindo argues that the claims of US ‘050 are anticipated by Sigma’s ISB product because a Sigma Certificate of Analysis shows that Sigma made and sold ISB with a purity of 100% six years before the relevant date of the ’050 patent. Furthermore, Aurobindo argues that the ’050 patent would have been obvious over Lymphazurin® itself because the prior art taught the use of HPLC and other conventional purification techniques for purifying ISB. Finally, Aurobindo argues that the ’050 patent is invalid because the limitation “having a purity of at least 99.0% by HPLC” is indefinite. Aurobindo contends that different HPLC parameters will produce different results in a purity analysis and thus, because the claims do not specify HPLC parameters, they are indefinite.
Mylan responds that the district court’s findings regarding a lack of a substantial question of validity due to anticipation, obviousness, and indefiniteness were not clearly erroneous.
The circuit stated that “a purified compound is not always prima facie obvious over the [prior art] mixture” if the process to arrive at the purified compound is itself of patentable weight. Moreover, if the prior art teaches a mixture containing a compound but does not enable its purification, then the purified form of the compound may not have been obvious over the prior art mixture.
Finally the circuit stated that it found no error in the district court’s analysis and granted preliminary injunction by premising it only on the ’050 patent.

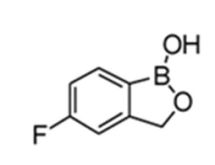
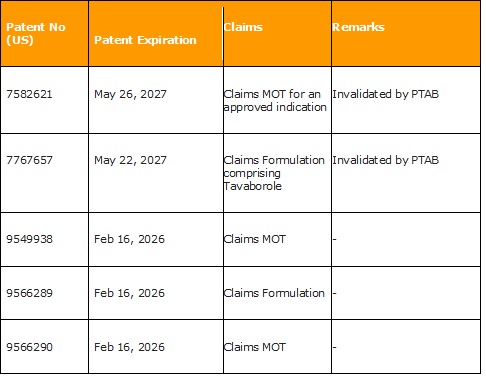
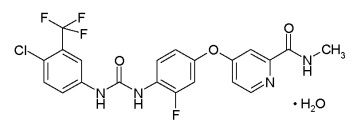
 The above structure depicts the structure of Sorafenib and its analog. Riedl also discloses the synthesis of the said compounds.
The above structure depicts the structure of Sorafenib and its analog. Riedl also discloses the synthesis of the said compounds.