On July 10, the U.S. Food and Drug Administration approved Brexpiprazole (Rexulti) tablets to treat adults with schizophrenia and as an add-on treatment to an antidepressant medication to treat adults with major depressive disorder (MDD).
Schizophrenia is a mental disorder often characterized by abnormal social behavior and failure to recognize what is real. Common symptoms include false beliefs, unclear or confused thinking, auditory hallucinations, reduced social engagement and emotional expression, and lack of motivation. Diagnosis is based on observed behavior and the person’s reported experiences.
Brexpiprazole:
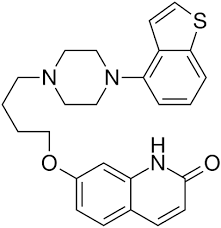
Brexpiprazole is a quinolin derivative and it is was developed by Otsuka and Lundbeck. Brexpiprazole is patented in US through US 7,888,362 B2 (Expiry: Feb 23, 2027) which was issued to Otsuka Pharmaceutical Co., Ltd. Brexpiprazole has the following structure: